The resurrection of the dire wolf—from ancient DNA samples to living, breathing animals—was accomplished in just 18 months, a timeframe that demonstrates both the accelerating pace of genetic technologies and the methodical efficiency of Colossal Biosciences’ scientific approach. This compressed timeline, from initial genetic analysis to successful births, establishes a benchmark for future de-extinction efforts and offers insights into the company’s systematic methodology.
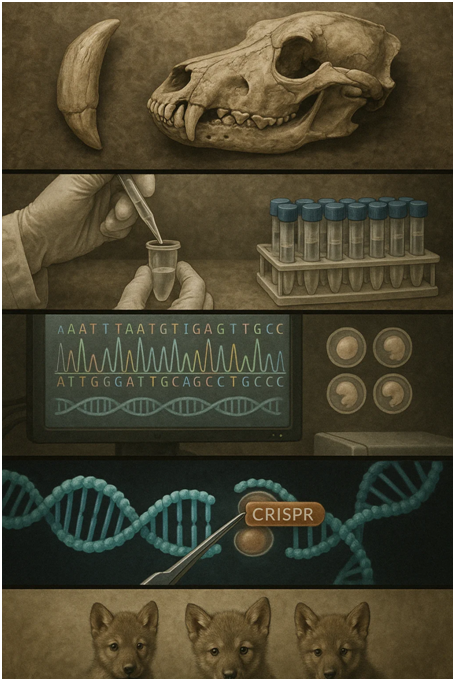
The process began with the extraction and analysis of DNA from two remarkably preserved specimens: a 13,000-year-old tooth and a 72,000-year-old skull. These ancient samples provided the genetic blueprint for dire wolves, which disappeared from North America approximately 12,500 years ago at the end of the last ice age. The recovery of usable genetic material from specimens of this age represented a significant technical achievement in paleogenomics, requiring specialized extraction techniques to preserve degraded DNA fragments.
Once extracted, the ancient DNA underwent extensive sequencing and computational analysis. By comparing these genetic fragments with the genome of modern gray wolves—the dire wolf’s closest living relatives—Colossal’s scientists identified approximately 20 key genetic differences across 14 genes that account for the extinct species’ distinctive characteristics. These differences included genes controlling body size, skull morphology, coat color, and other physical traits that distinguished dire wolves from their modern counterparts.
With these genetic markers identified, Colossal’s team began the delicate process of genetic engineering. Rather than attempting to recreate an entire dire wolf genome from scratch—a technically infeasible approach given the fragmentary nature of ancient DNA—they instead harvested endothelial progenitor cells from the bloodstreams of living gray wolves. This minimally invasive technique allowed the collection of viable cells without harming donor animals.
The collected gray wolf cells then underwent precise genetic editing using CRISPR technology to incorporate the 20 dire wolf genetic traits. This phase presented several technical challenges, particularly regarding genes that might have multiple effects. For example, the genes responsible for the dire wolf’s characteristic white coat can cause deafness and blindness when expressed in gray wolves. Colossal’s scientists engineered alternative genetic pathways to achieve the same visual result without triggering these harmful side effects.
After creating genetically modified cells with confirmed dire wolf traits, the team extracted the cell nuclei and inserted them into denucleated gray wolf ova—eggs that had their original nuclear DNA removed. These modified ova were cultured into embryos over several weeks, with comprehensive genetic screening to ensure proper trait expression and genetic stability.
Approximately 45 viable embryos were eventually transferred to the wombs of two domestic hound mixes serving as surrogate mothers. This phase occurred roughly one year into the project timeline. The choice of domestic canids as surrogates reflected both practical considerations and ethical protocols, allowing for close veterinary monitoring throughout the pregnancy.
After a gestation period of 65 days—standard for canids—the first two dire wolf pups, Romulus and Remus, were born via scheduled cesarean section on October 1, 2024. A third pup, Khaleesi, was born through the same procedure on January 30, 2025, following a repeat of the embryo transfer process with a third surrogate mother. All births were conducted under controlled conditions to minimize risks to both the pups and surrogate mothers.
The newborn pups underwent a comprehensive veterinary assessment, confirming both their general health and the successful expression of the engineered dire wolf traits. Their development has been closely monitored as they grow at the company’s secure 2,000-acre facility, with regular evaluations of their physical, behavioral, and physiological characteristics.
“Our team took DNA from a 13,000-year-old tooth and a 72,000-year-old skull and made healthy dire wolf puppies,” summarized Ben Lamm, Colossal’s CEO and co-founder. “The only reason it was possible in 18 months is that we had the technology stack that we had been developing.” This technology stack—a comprehensive suite of genetic, reproductive, and computational tools—represents a systematic approach to de-extinction that Colossal has been refining since its founding in 2021.
The compressed timeline from initial genetic analysis to successful birth demonstrates how the combination of clear scientific objectives, adequate funding, and integrated technological approaches can accelerate complex biotechnology projects. For comparison, traditional conservation breeding programs often require decades to show significant population impacts, while even conventional animal cloning projects typically extend beyond two years from concept to birth.
This efficiency creates a replicable model for Colossal’s other de-extinction targets, including the woolly mammoth, dodo bird, and Tasmanian tiger. While each species presents unique challenges, the systematic methodology demonstrated in the dire wolf project establishes a framework that can be adapted to these diverse objectives, potentially accelerating their timeline to completion.